Nuvaxovid
Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Contact your healthcare professional if you have any questions about the product.

Fda Advisers Back Authorization Of Novavax Covid Shot Medpage Today
About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes.
. Nuvaxovid dispersion for injection. Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. Nuvaxovid Novavax is approved and available for use as a primary course in people aged 12 years and over.
Company Novavax should not be given to individuals younger than 30 the Public Health Agency of. Det eftersom att data från Australien gett. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19.
Nu stoppar Folkhälsomyndigheten användningen bland personer. Qualitative and quantitative composition. Rokotteesta ei myöskään ole haittaa vaikka.
First Approval of the Protein-Based Adjuvanted Nuvaxovid NVX-CoV2373 Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide. COVID-19 Vaccine recombinant adjuvanted 2. Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic.
Nuvaxovid-rokote sopii lähes kaikille aikuisille. Nuvaxovid is the first protein-based COVID-19 vaccine granted. Vial and carton labels with English-only labelling.
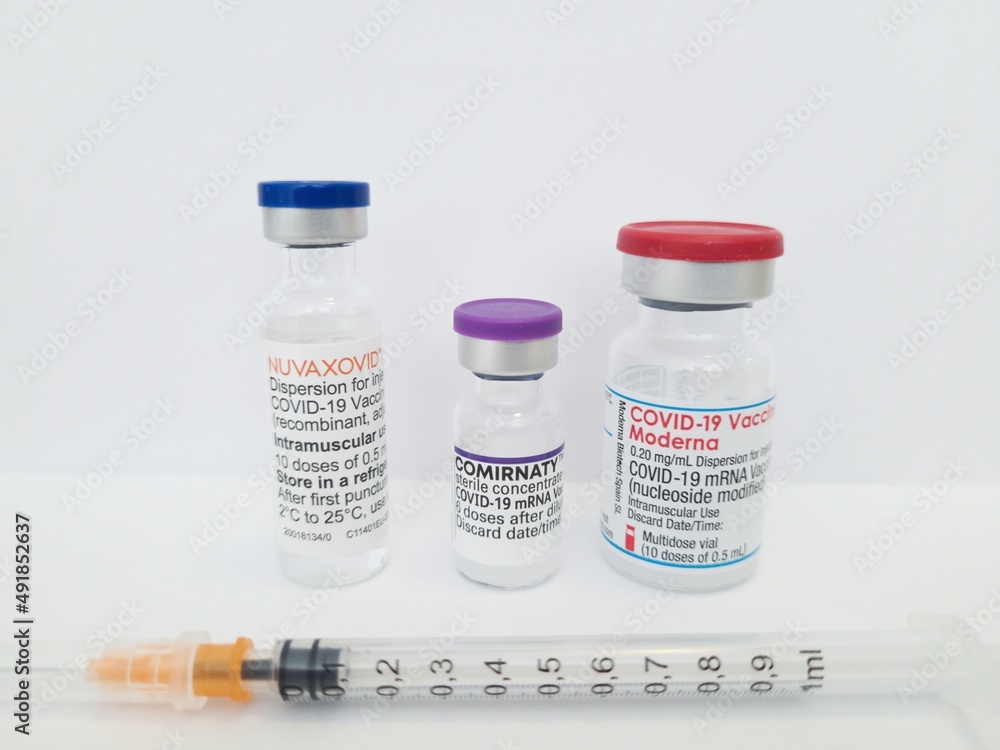
Nuvaxovid is packaged as a ready-to-use liquid formulation in a vial containing ten doses. Clinical trials showed that the vaccine has around 90 efficacy. The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency.
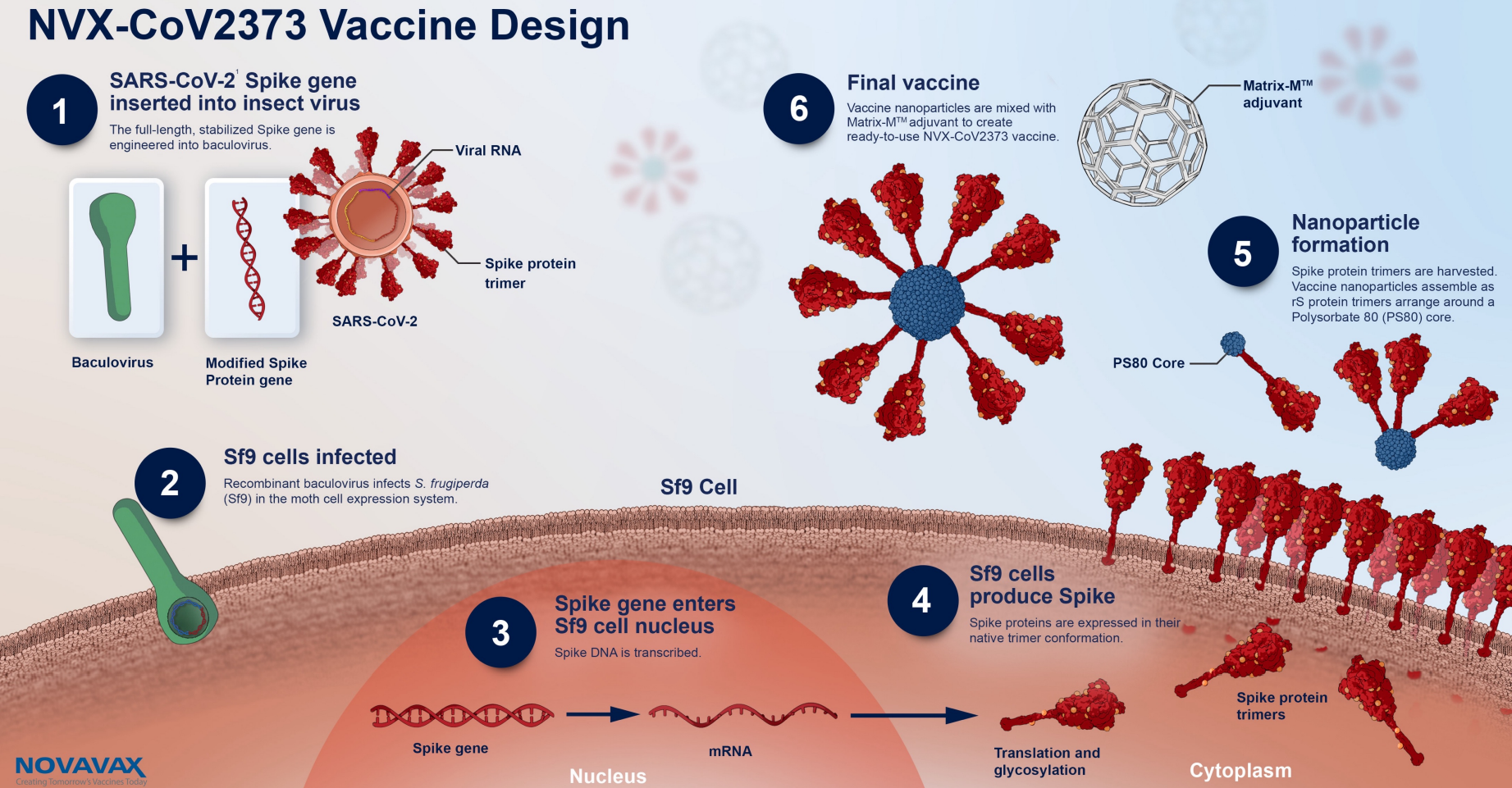
Vaccinationer med Nuvaxovid pausas för personer 30 år och yngre Folkhälsomyndigheten. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. 2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US.
Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. Like the Novavax vaccine side effects were more. 3 hours agoNovember 2 2022 Famagusta Gazette Covid Europe Sweden 0.
Beslutet är temporärt och gäller från. Enligt testerna skulle det ju vara säkrare än Pfizer hur kan det komma sig att Pfizer används. The Summary of Product Characteristics is a description of a.
The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further. This is a multidose vial.
On December 20 2021 the. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. 5 hours agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen.
88 experienced pain. Nuvaxovid the COVID-19 vaccine created by US. The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19.
16 fever including 14 severe cases. Company Novavax should not be given to individuals. Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022.
6 hours agoDet proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Name of the medicinal product. Novavax is approved and available for use as a booster in.

Fda Advisers Overwhelmingly Endorse Novavax Covid 19 Vaccine Ars Technica
Novavax S Covid 19 Vaccine Nuvaxovid Gets Conditional Approval In Switzerland Seeking Alpha

Novavax Nuvaxovid Covid 19 Vaccine Star Pharmacy

Novavax S Vaccine Nuvaxovid Vaktsineeri Ee
Nuvaxovid Otazky A Odpovedi K Vakcine Od Spolecnosti Novavax Kurzy Cz

Informacoes Sobre A Vacina Nuvaxovid Novavax Contra Covid 19 Australian Government Department Of Health And Aged Care

Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Gov Uk
Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022

Eu Regulator Backs Use Of Novavax Covid Shot As A Booster
Allegheny County Health Department Will Start Administering Novavax Vaccine Pittsburgh Post Gazette

Nuvaxovid Novavax Covid 19 Vaccine Course The Immunisation Advisory Centre

Novavax Covid Vaccine Nuvaxovid Medcity News

Nuvaxovid Novavax Covid 19 معلومات در باره واکسین Australian Government Department Of Health And Aged Care

Informacie O Covid 19 Nuvaxovid Novavax Vakcine Australian Government Department Of Health And Aged Care
Switzerland Approves First Protein Based Covid Vaccine

Nuvaxovid Novavax S Covid 19 Vaccine Approved For 18y And Older The Immunisation Advisory Centre

Tga Provisionally Approves Covid 19 Vaccine Nuvaxovid For Use In 12

Middlesex London Health Unit Are You Interested In The Novavax Nuvaxovid Covid 19 Vaccine Mlhu Is Receiving A Limited Supply Of The Vaccine This Spring Call 1 226 289 3560 To Be Added To The Waitlist
